Background: Overexpressionof B-cell lymphoma 2 (BCL-2)-related pro-survival proteins allows cancer cells to evade apoptosis, and targeting these proteins has become important in anticancer therapy. BCL-2 inhibition by venetoclax (VEN) is beneficial in many patients (pts) with acute myeloid leukemia (AML), but resistance is common due to upregulation of other pro-survival proteins such as BCL-extra-large (BCL-xL) and myeloid cell leukemia-1. Dual inhibition of BCL-2/xL has potential for broader activity than agents such as VEN. However, BCL-xL is critical for platelet survival and inhibition can cause dose-limiting thrombocytopenia (Konopleva and Letai. Blood 2018). To broaden therapeutic activity, we developed AZD0466, a drug-dendrimer conjugate in which the BCL-2/xL dual inhibitor AZD4320 is covalently conjugated to a pegylated poly-L-lysine dendrimer and gradually released by hydrolysis. Preclinically, AZD4320 showed greater inhibition of tumor growth than VEN and navitoclax in VEN-resistant xenograft models (Balachander et al. Clin Cancer Res 2020). A first-in-human study (NCT04214093) in pts with advanced solid tumors indicated that AZD0466 is well tolerated with no reported dose-limiting toxicities (DLTs). We present preliminary data from NIMBLE, a Phase I/II trial of AZD0466 in pts with advanced hematologic malignancies (NCT04865419).
Methods: Module 1, Part A of NIMBLE is a dose-escalation study of AZD0466 monotherapy investigating its preliminary safety and tolerability, pharmacokinetics (PK), and preliminary efficacy. Dose escalation and de-escalation of AZD0466 follow a modified toxicity probability interval (mTPI-2) design. Eligible pts are ≥18 years old with relapsed/refractory (R/R) AML, acute lymphoblastic leukemia (ALL), or high-risk myelodysplastic syndrome. AZD0466 is administered IV with a dose ramp-up on Days (D) 1, 4, and 8, reaching the target dose on D8 of Cycle 1, with weekly administrations thereafter. Cycle duration is 35 days for Cycle 1 and 28 days for subsequent cycles. Pts remain on study until disease progression, unacceptable toxicity, or withdrawal of consent.
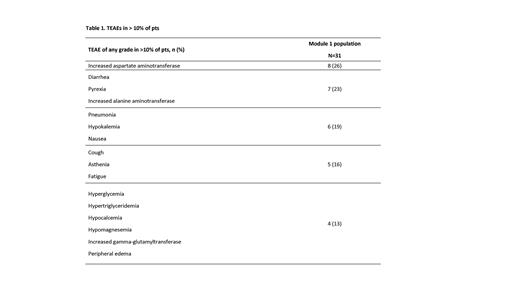
Resul ts: As of May 31, 2023, 31 pts (n=25 AML, n=6 ALL; median age 69 years) had received ≥1 dose of AZD0466 at target doses of 300 mg (n=4), 600 mg (n=4), 1200 mg (n=7), 2400 mg (n=5), 3600 mg (n=9), and 5400 mg (n=2). Median treatment duration was 4.1 weeks (range 0.1−29.6). Twenty-two pts (71%) had prior VEN and 11 (35%) had prior hematopoietic stem cell transplantation. Treatment-emergent adverse events (TEAEs) of any grade reported in >10% of pts are displayed in Table 1. One Grade 5 intracranial hemorrhage unrelated to AZD0466 was reported. Four pts experienced a serious adverse event (SAE) possibly related to AZD0466 (alanine aminotransferase [ALT] increase, pneumonia, troponin T increase, and uncoded SAE). Grade ≥3 TEAEs possibly related to AZD0466, reported in 12 pts (39%), included febrile neutropenia (n=3), platelet count decrease (n=2), diarrhea, thrombocytopenia, ALT increase, blood creatinine phosphokinase increase, pneumonia, gamma-glutamyltransferase increase, lipase increased, and troponin T increase (n=1, each). DLTs of asymptomatic troponin increase were observed in two pts (3600 mg [n=1], 5400 mg [n=1]). Evaluation of maximum concentration and area under the concentration-time curve revealed dose-proportional exposure to AZD4320. Released AZD4320 represented 1-5% of total AZD4320 and had a longer half-life than total AZD4320. Preclinical modeling and PK data predicted that 5400 mg may be required to drive efficacy. As a surrogate of BCL-xL on-target activity, transient aggravation of thrombocytopenia post-dosing was observed across all dose cohorts followed by platelet recovery prior to the next dose. Platelet reduction was in line with preclinical models showing a substantial reduction at lower doses with a ceiling effect at higher doses. As of July 6, 2023, no complete responses have been observed. However, preliminary bone marrow (BM) blast reductions of >50% were observed in four pts (1200 mg [n=1], 2400 mg [n=2], 3600 mg [n=1]), indicating antileukemia activity of AZD0466.
Conclusions: Given the observed DLTs of asymptomatic increase in troponin levels, and the lack of significant clinical benefit, the study was terminated.
OffLabel Disclosure:
Fleming:Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Curti:Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Dinner:Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Rigel: Research Funding; Kite/Gilead: Research Funding. Blachly:Abbvie: Honoraria; Astellas: Honoraria; AstraZeneca: Honoraria; Kite: Honoraria. Della Porta:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bajel:Novartis: Honoraria; Astellas: Honoraria; Amgen: Honoraria, Speakers Bureau; Pfizer: Honoraria; Abbvie: Honoraria. Sauer:Stemline: Consultancy; Novartis: Consultancy; Ridgeline Discovery: Consultancy; BMS: Consultancy; Amgen: Consultancy; Astellas: Consultancy; Takeda: Consultancy; AbbVie: Consultancy, Other: Travel, Accommodation, Expenses; Jazz: Honoraria, Other: Travel, Accommodation, Expenses; Gilead: Honoraria; Pfizer: Honoraria. Crysandt:Pfizer: Other: Travel grant. Pigneux:Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings; Gilead: Honoraria; Roche: Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees. Fransecky:Pfizer: Consultancy; Jazz: Consultancy; Gilead: Consultancy, Research Funding, Speakers Bureau; Servier: Consultancy; Amgen: Consultancy. Baldus:BMS: Consultancy; AstraZeneca: Consultancy; Amgen: Consultancy; Astellas: Consultancy; Jazz Pharmaceuticals: Consultancy; Gilead: Consultancy; Jannsen: Consultancy. Konopleva:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trials Support, Research Funding. Marmor:AstraZeneca: Current Employment, Current holder of stock options in a privately-held company. Sharma:AstraZeneca: Current Employment. Elgeioushi:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Cheung:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Rowe:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Jahn:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Yu:AstraZeneca: Current Employment, Current equity holder in publicly-traded company; JNJ: Current equity holder in publicly-traded company; AbbVie: Current equity holder in publicly-traded company; Abbott: Current equity holder in publicly-traded company. Saeh:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Tibes:AstraZeneca: Current Employment. Cader:AstraZeneca: Current Employment. Jain:Aprea Therapeutics: Research Funding; Dialectic Therapeutics: Research Funding; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Fate Therapeutics: Research Funding; Incyte: Research Funding; TransThera Sciences: Research Funding; Medisix: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pfizer: Research Funding; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Takeda: Research Funding; ADC Therapeutics: Research Funding; Servier: Research Funding; MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Novalgen: Research Funding; Mingsight: Research Funding; Loxo Oncology: Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Newave: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding.
AZD0466 is a drug-dendrimer conjugate containing a BCL-2/xL dual inhibitor that is in development for use in hematological and other malignancies, and not approved for use anywhere worldwide

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal